pH Imbalance - Getting to the Root of the Problem
Science is more meaningful and engaging when students have the opportunity to explore the world around them. And it is more meaningful still when students are empowered with the tools to collect and analyze data just like real scientists do, allowing hypotheses to be tested and conclusions to be drawn. As citizen-scientists, we see the science everywhere— even in our own backyard. In this case, we had a citrus tree that was not doing so well, and we used this opportunity to investigate and try to identify the cause of our ailing tree to try to resolve the issue.

You can probably guess that yellow lemons are great, but yellowing leaves are not. One cause of leaf yellowing can be the poor soil pH. If the pH is outside the optimal range of about 5-6.5, then the nutrients will not be available to the plants roots, resulting in the yellowing leaves we can see. A knee jerk reaction to a plant that does not appear to be flourishing is to provide fertilizer, but if the pH balance is off, additional fertilizer will not help.
So how could we find out if it was a pH issue? By getting some dirt under our fingernails and collecting some data. The best way to measure the soil pH is to take a sample and mix it with water, so we created a slurry sample in our impromptu lab. After the slurry settled, it was time to test the pH.

Using the wireless pH sensor and SPARKvue software on a phone, we took a quick measurement of the pH of the soil sample.
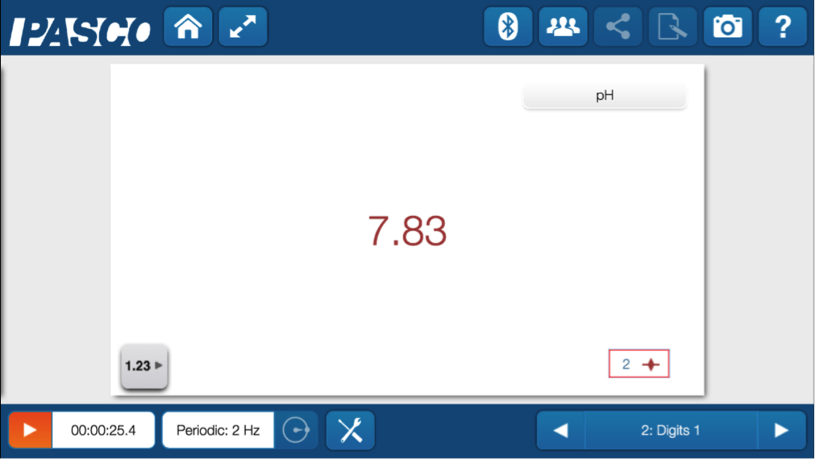
The pH was a bit high indicating that the soil was alkaline. We know we can address this by adding sulfur to the soil to bring down the pH.
Wireless sensors and SPARKvue enable students to easily explore and experiment with the world around them. The fruits of their labors will be conceptual understanding of acids, bases and soil pH— and hopefully some delicious fruit!


