Applications of Titrations
Learn how titration helps industries maintain quality standards, optimize production processes, and more in this short blog!
What Are the Applications of Titrations?
Titration is an analytical method commonly used to determine the concentration of a solution. However, titrations also have applications in the pharmaceutical industry, food industry, and more.

Study of the Reaction Kinetics of Chemical Reactions
Reaction kinetics is the study of how chemical reactions occur over time, including the rate of reaction, the mechanism of how the reaction occurs, and other influential factors like temperature and concentration. Titrations can be used to study these factors by measuring the rate of the reaction in real time.
The rate of the reaction is determined by monitoring the change in pH during the titration. The pH of the analyte changes as the acid or base is added, and when the solution becomes neutral, the pH levels out.
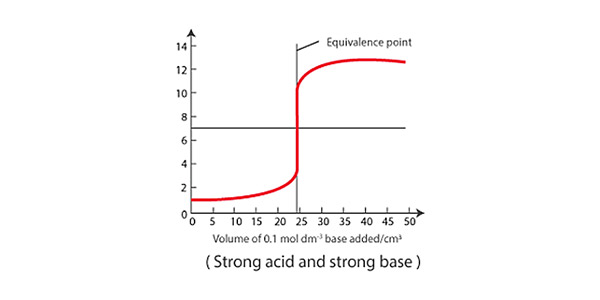
During a titration, the experimenter records both the volume of titrant used and the pH of the analyte, which allows them to create a pH curve to determine the reaction rate of the chemical reaction taking place. Steeper slopes at the early stages of titration indicate faster reactions, and vice versa. Generally, the pH will change rapidly in the beginning as the titrant first reacts with the acid or base, and then change more slowly as the endpoint of the titration is reached. In the lab, this information can be used to optimize reaction conditions and design more efficient chemical processes.
Teaching Tools: Wireless pH Sensor
An essential for any science course, the Wireless pH Sensor streams data wirelessly to your device for a wide range of applications. Investigate concepts in chemistry, biology, and environmental science confidently with long battery life and an onboard memory that accommodates both short or long-term studies.

| Learn More |
Purification of Chemicals
Titrations can remove a variety of impurities from solutions depending on the method used. For instance, acid-base titrations can selectively neutralize excess salts, metals, acids, or bases. They can also be used to measure a solution’s acid or base content, which can then be compared to the expected value for a pure sample. This information is valuable for ensuring the quality of products and for meeting regulatory requirements in industries such as food production and environmental monitoring, which we’ll discuss in more detail below.
Redox titrations can oxidize or reduce excess organic compounds, as well as reduce oxides to a less reactive form. Lastly, precipitation titrations remove halides by precipitating them as insoluble salt which is subsequently filtered out of the solution.

Analysis of Food Products
Redox titrations, in particular, are commonly used in the quality control of food products to determine the levels of antioxidants and oxidants present in food. The oxidation of fats and oils in our food leads to spoilage; therefore, researchers titrate random samples of product to determine the amount of antioxidants needed to prevent oxidation and increase shelf-life and food quality.

A common method for measuring the antioxidant capacity of food products is known as the oxygen radical absorbance capacity (ORAC) assay, which uses a redox indicator to measure the ability of the sample to scavenge free radicals that cause oxidation, typically in fruits and vegetables.
The indicator, fluorescein, is a fluorescent dye that loses its fluorescence when oxidized. Free radicals—highly reactive atoms or molecules—easily oxidize fluorescein. However, free radicals also readily react with antioxidants. Thus, the presence of antioxidants in the sample interrupts the oxidation of fluorescein, extending the duration of the fluorescein’s glow. By monitoring the reaction over time, researchers can calculate the antioxidant capacity of the sample based on the rate that fluorescein loses its glow.
Because free radicals are also present in our body, ORAC assays are also used to study the health benefits of antioxidants and to develop new antioxidant-rich foods and health supplements.
Teaching Tools: Wireless Drop Counter
|
Perform titrations easier with the Wireless Drop Counter! The included drop dispenser and rod clamp make titration setups a breeze, while the wider drop window ensures you’ll never miss a drop of titrant again.
|
|
| Learn More |
Quality Control
Titration is necessary to check for the correct concentration of ingredients to ensure the quality of products like pharmaceuticals, soap, and even biodiesel fuel.
In quality control, titration provides a method of removing impurities from a sample by selectively reacting with the impurities, as we discussed earlier. For example, in the pharmaceutical industry, acid-base titrations can be used to purify crude drugs—drugs derived from nature—by selectively reacting titrants with impurities such as salts, metals, and other contaminants.
In this process, a sample of the crude drug is dissolved in a solvent, and an acid or base is added to the solution. The acid or base reacts with the impurities in the solution, forming a precipitate that can easily be removed by filtration. After the removal of the impurities, the pH is adjusted back to its original value, and the pure drug can be recovered by evaporating the solvent.
Drug Titration
Drug titration is the process of adjusting the dosage of a medication to achieve a desired therapeutic effect. This result is achieved through a tad of trial and error, in which the medical provider gradually adjusts the dosage until the patient’s response is optimal. The goal is to find the lowest effective dose while minimizing side effects for the patient.

This type of titration is commonly used to treat chronic conditions like hypertension, diabetes, and depression—illnesses that require dose adjustments over time. Healthcare professionals must monitor the patient’s vital signs, blood glucose levels, or other variables to weigh the treatment response and adjust the medication dosage as needed.
Utilizing titration in patient care allows healthcare providers to tailor treatment to the specific needs of each patient, reduce medication errors, and remove ineffective treatments.
Environmental Monitoring
Titrations are commonly used to measure the concentration of various chemical species in water, soil, and air samples. For example, acid-base titrations are used to measure the alkalinity and acidity of water samples to determine the presence of pollutants like acid rain, industrial discharges, and more.

Other types of titrations can measure the concentration of heavy metals, pesticides, and organic compounds in environmental samples; researchers use this data to monitor environmental regulation compliance in the area and identify sources of contamination.
In environmental monitoring, titration results are used to make decisions about land use, resource management, and environmental protection, and to develop strategies to minimize the impact of pollution on human health and the environment.