The Chemistry of Water
What exactly is water? Why is it an amazing solvent? Is it an acid or base? We answer these questions and more in this short blog!
Water makes up 71% of the Earth’s surface, up to 4% of our atmosphere, and about 60% of our body. It is the only substance on Earth that exists readily in all three states of matter: solid, liquid, and gas. It’s a simple compound with just three atoms, yet the chemistry of water makes it vital for all living things.

Is Water an Acid or Base?
Water has a pH of 7, so it’s neither an acid nor a base by definition. Rather, water is an amphoteric species, which means it can act as an acid or a base. The behavior of water during an acid-base reaction—that is whether it acts as an acid or a base—is determined by the other species involved in the reaction.
Water in Acid-Base Reactions
Water behaves as a base when reacting with an acid.
HA + H2O ⇌ H3O+ + A-
Water behaves as an acid when reacting with a base.
H2O + B- ⇌ HB + OH-
As an amphoteric compound, water reacts with itself through a process called autoionization or self-ionization. The autoionization reaction of water is shown below:
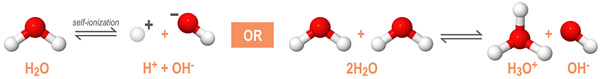
In a solution of pure water, two water molecules will react to produce equal concentrations of hydronium (H3O+) and hydroxide (OH-) ions. In an acid-base reaction, the concentration of these ions is determined by the temperature and the pH of the solution.
Note: the hydronium ion (H3O+) and hydrogen ion (H+) are often used interchangeably to represent the hydrated proton in an acid-base reaction.
In the example below, ammonia (NH3) accepts protons from the surrounding water molecules, reducing the concentration of hydrogen (H+) ions present in the solution. Without as many hydrogen ions to bond, an excess of hydroxide (OH-) ions form in the solution.
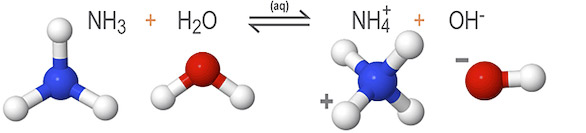
Here, water dissociates to form an excess of hydroxide ions (OH-), making it an Arrhenius base. But it also donates protons to ammonia (NH3), making it a Bronsted-Lowry acid. This reaction is unique in that both reactants—ammonia and water—are amphoteric.
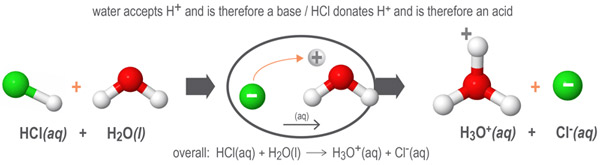
In this next example, water accepts protons from the hydrogen chloride HCl molecules to form its conjugate acid, the hydronium ion H3O+. When water accepts protons, it acts as a Bronsted-Lowry base.
Teaching Tools: Wireless pH Sensor
Monitor pH during acid-base titrations, explore buffers, and investigate water quality with the Wireless pH Sensor. Log pH directly onto the sensor for long-term experiments, or wireless connect it to your device to stream data in real time!

| Learn More |
Water Polarity
Water is unique in that it’s able to act as both a solvent and a reactant, making it an ideal medium for many reactions. It’s also polar, which allows it to dissolve solutes involving polar and ionic compounds.
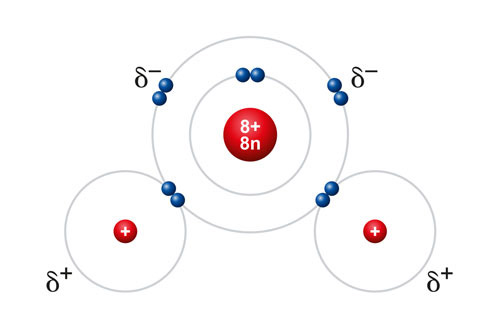
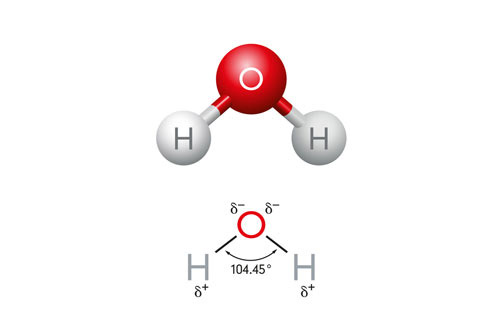
Polarity refers to the distribution of electric charge within a molecule, creating a positive end and a negative end. In the case of water molecules, there is unequal sharing of electrons between the oxygen and hydrogen atoms, resulting in a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. This polarity allows water to interact with other charged molecules, making it an excellent solvent – you may have even heard water described as a “universal solvent” for this ability.
Structure of Water and Hydrogen Bonds
The hydrogen bonds in water enable it to participate in many chemical reactions such as acid-base and hydrolysis reactions. Hydrogen bonding is a type of intermolecular bond between a positively charged hydrogen atom and an electronegative atom in a nearby molecule.
 |
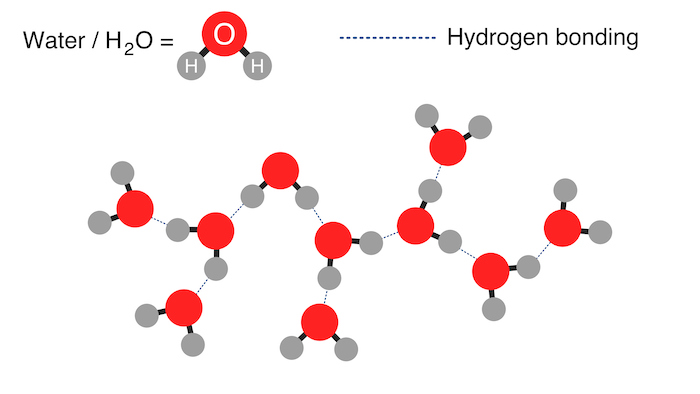 |
In water, the molecules orient themselves so the hydrogen bonds form between the partially positive hydrogen atoms of one molecule and the partially negative oxygen atoms of nearby water molecules. This bonding between water molecules is responsible for many of its qualities, including its high boiling point, surface tension, and ability to dissolve many substances.
Teaching Tools: Wireless Temperature Sensor
Equip your chemistry students with the only thermometer they'll ever need! Durable and easy to use, the Wireless Temperature Sensor connects to your device via Bluetooth, making it easy to monitor, plot, and record temperatures in real time.

| Learn More |
Water has a high boiling point (100°C, 212°F at 1 atm), meaning that a significant amount of heat energy is required to increase its temperature. This makes it an excellent thermoregulator in both controlled experiments and the natural environment. In fact, water’s ability to buffer changes in temperature plays an essential role in biological systems, from maintaining homeostasis to regulating heat in the oceans.

Water’s versatile nature and amphoteric properties make it an essential component of many chemical reactions; understanding its role in these processes is crucial for many fields of science, including biology, chemistry, and environmental science.
Download our free lab, Properties of Water, for a fun way to study the molecular properties of water in your classroom! Or, check out our entire library of chemistry, physics, and environmental labs here!
