Acid and Base Strength
Learn the fundamentals of acids and bases and how to calculate their strength using dissociation constants.

Fundamentals of Acids and Bases
Ka and pKa are two terms that are used to describe the strength of acids. An acid is a substance that releases hydrogen ions (H+) into solution, or “donates” hydrogens, according to the Bronsted-Lowry definition.
Kb and pKb , on the other hand, describe the strength of bases. A base is a substance that accepts hydrogen ions (H+) in a solution.
To jump to the section on common strong acids and bases, click here.
The conjugate acid of a base is a substance that forms when the base accepts a hydrogen ion. The conjugate base of an acid is a substance that forms when the acid releases a hydrogen ion.
Note: The stronger the acid, the weaker the conjugate base. The weaker the acid, the stronger the conjugate base.
Consider the reaction below of acid and water. The strength of the acid (HA) determines how well it ionizes in water (H2O) to produce hydronium ions (H3O+) and a conjugate base (A-).
HA(aq) + H2O(l) ⇌ H3O+(aq) + A-(aq)
Teaching Tools: Wireless Conductivity Sensor
Perform conductivity titrations, compare acid and base strength, and more with the Wireless Conductivity Sensor. This sensor is dust-proof, sand-proof, and water-resistant, making it ideal for studies inside or outside the classroom!

| Learn More |
Acid Dissociation Constant Ka
The strength or weakness of an acid or base depends on the compound’s capacity to dissociate in solution. In aqueous solutions, strong acids and bases dissociate completely into their component ions, while weak acids and bases only dissociate partially. Ka is the equilibrium constant for the dissociation of an acid, and is sometimes referred to as the acid ionization constant. It is defined by the expression:
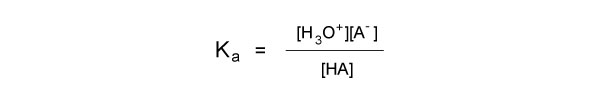
Ka is a measure of how easily an acid gives up its hydrogen ions. The larger the value, the stronger the acid, and the more it will dissociate in solution. The lower the Ka value, the weaker the acid, and the less it will dissociate in solution.
Further, weak acids have Ka values less than 1. Strong acids have a Ka value greater than 1.
| Weak Acids | Strong Acids |
| Ka < 1 | Ka > 1 |
| Do not readily dissociate in solution | Readily dissociate in solution |
| Lower [H+] at equilibrium | Higher [H+] at equilibrium |
| Equilibrium lies to the left | Equilibrium lies to the right |
You can use the acid dissociation constant to find the concentration of H+ present in a solution at equilibrium. The concentration varies depending on the type of acid used.
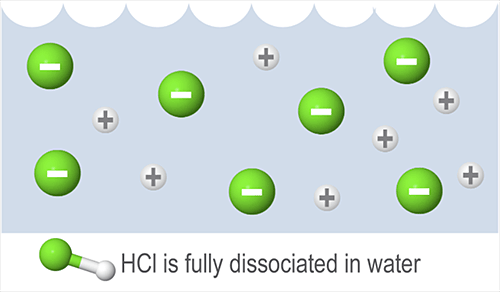
Hydrochloric acid has a Ka of 1.3102, so it ionizes completely in water. For this reason, the reaction equilibrium falls far to the right. The equilibrium can be indicated by the size and direction of the arrow in the equation. In this case, all the reactants will turn into products, and the rate of the reverse reaction is nearly zero, so a one-directional arrow indicating a forward reaction is used:
HCl + H2O → Cl- + H3O+
However, when a weak acid is added to water, the solution enters an equilibrium state in which aqueous acid molecules (HA) react with liquid water to form aqueous hydronium ions (H3O+) and aqueous anions (A-). The reaction is reversible. This is described by the expression:
HA(aq) + H2O(l) ⇌ H3O+(aq) + A-(aq)
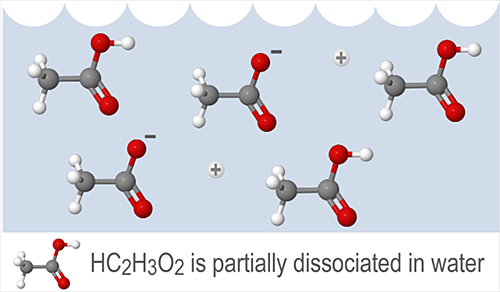
For example, citric acid (C6H8O7) and acetic acid (HC2H3O2) are both weak acids.
Ka = 7.410-4 for citric acid and Ka = 1.810-5 for acetic acid. The Ka of acetic acid is less than that of citric acid, which tells us the following:
- Citric acid is the stronger acid of the two.
- At equilibrium, aqueous citric acid dissociates more fully than aqueous acetic acid.
In the reaction below, acetic acid is ionized to form hydronium and acetate ions. Because acetic acid has a Ka value less than one, the reaction runs in both directions.
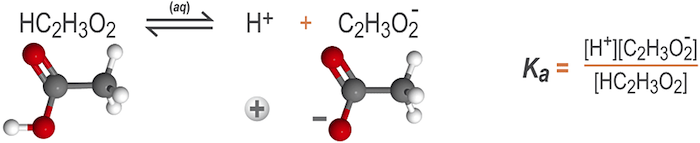
How is Ka calculated?
To calculate Ka, you need to know the concentrations of the hydronium ions, the conjugate base, and the acid. You can measure these concentrations using a variety of methods, such as titration or spectroscopy, and using the equation for the acid dissociation constant.
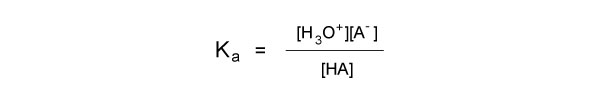
[H+] is the concentration of hydronium ions, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the acid.
Once you have the concentrations, you can plug them into the equation and solve for Ka.
Let’s consider the same example with hydrochloric acid to solve for Ka:
HCl + H2O → Cl-+ H3O+
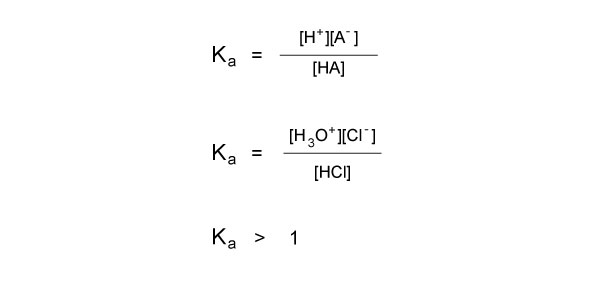
Since we know the equilibrium falls far to the right, we can infer that the concentrations of our conjugate base and conjugate acid are much larger than the concentration of HCl. Therefore, the numerator in the equation is much greater than the denominator, so we know that the Ka will be much greater than 1.
Let’s try an example with acetic acid (CH3COOH), a weak acid:
CH3COOH + H2O ⇌ CH3COO- + H3O+
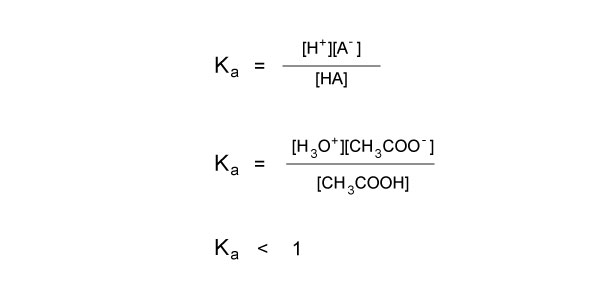
Because acetic acid is a weak acid, not all of it will dissociate in water. In fact, the equilibrium falls to the left. This tells us that the concentrations in the numerator are lower than the concentration in the denominator, so the Ka is a fraction.
pKa and Ka
The relationship between pKa and Ka is inverse, as shown in the equations below. The lower the pKa, the larger the Ka, and the stronger the acid.
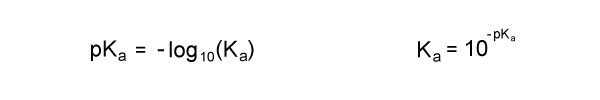
For example, a pKa of 2 means that the acid is 100 times stronger than an acid with a pKa of 3.
Why use pKa?
pKa is a more convenient way to express the strength of an acid. The pKa scale ranges from 0 to 14, with lower values indicating stronger acids. This makes it easy to compare the strengths of different acids.
The pKa scale is also useful for predicting the pH of solutions. The pH of a solution is a measure of how acidic or basic it is. For more information on pH and pOH, visit our Acid-Base Chemistry Product Guide.
The pH is calculated using the Henderson Hasselbalch Equation:
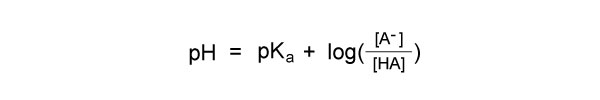
[A-] is the concentration of the acid, and [HA] is the concentration of the conjugate base.
By knowing the pKa of an acid, scientists can predict the pH of a solution that contains that acid. This information can be used to adjust the pH of a solution to a desired value.
Teaching Tools: Wireless pH Sensor
Monitor pH for chemical reactions, acid-base titrations, water quality studies, and more with the Wireless pH Sensor. An essential tool for every science classroom, this sensor streams data wirelessly to your device for easy data collection and analysis. Conduct short-term experiments and stream data in real time, or log data for long-term environmental applications.

| Learn More |
Base Dissociation Constants Kb and pKb
Recall that bases are a species that is likely to accept protons in solution. Bases, like acids, have varying levels of strength. Kb is the equilibrium constant for the dissociation of a base in water, as shown below:
B(aq) + H2O(l) ⇌ BH(aq) + OH-(aq)
Kb is determined by the expression:
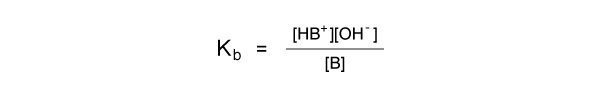
Stronger bases dissociate more easily in solution, so have a larger Kb value.
Kb and pKb are inverse, so a lower pKb indicates a stronger base.

The lower the Kb value, the weaker the base, and the less it will dissociate in solution.
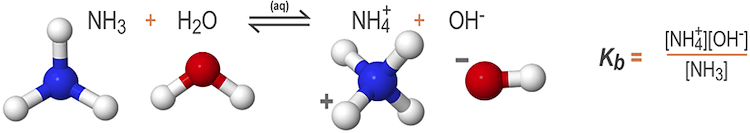
When a weak base is added to water, the compound dissociates partially and is protonated by the surrounding water molecules, forming aqueous base (HB+) and hydroxide (OH–) ions. The reaction is represented by the expression:
B(aq) + H2O(l) ⇌ HB+(aq) + OH-(aq)
How to Identify Acids and Bases as Strong or Weak
The 7 common strong acids include:
- Hydrochloric acid (HCl)
- Chloric acid (HClO3)
- Perchloric acid (HClO4)
- Hydrobromic acid (HBr)
- Hydroiodic acid (HI)
- Nitric Acid (HNO3)
- Sulfuric Acid (H2SO4)
The most frequently used strong acids are hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
The 7 common strong bases include:
- Lithium hydroxide (LiOH)
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)2)
- Strontium hydroxide (Sr(OH)2)
- Magnesium hydroxide (Mg(OH)2)
- Barium hydroxide (Ba(OH)2)
The most frequently used strong bases are sodium hydroxide (NaOH), potassium hydroxide (KOH), and lithium hydroxide (LiOH).
When given the full chemical equation:
- Count the hydrogens on each of the reactants and products. The reactant that loses one or more hydrogens is the acid. The substance that gains one or more hydrogens is the base.
- Determine whether the substance is strong or weak using the lists above.
When given a compound:
- Determine whether the substance is an Arrhenius acid or base.
- Does the name of the compound end in "acid"? If so, it's an acid.
- Does the chemical formula resemble: HX(aq) or HaXbOc_? If so, it's an acid.
- Does it contain basic anions such as hydroxide (OH-), carbonate (CO32-), or hydrogen carbonate (HCO32-)? If it contains basic anions or ammonia (NH3), it's a base.
- Determine whether the substance is strong or weak using the lists above.
Weak Acids and Bases
Weak acids and weak bases only dissociate partially in solution. Commonly used weak acids include acetic acid (CH3COOH), hydrofluoric acid (HF), and oxalic acid (COOH)2. Commonly used weak bases include ammonium hydroxide (NH4OH), and ammonia (NH3).
 |
 |
Kw, Ka, and Kb
Ka and Kb are related through the ionization constant for water, Kw.
Kw is a measure of the extent to which water molecules dissociate into hydrogen ions (H+) and hydroxide ions (OH-) in aqueous solution. It is defined as the product of the concentrations of these ions, as shown below:
Kw = 1.0 × 10-14
The ionization of water is an equilibrium reaction, and the value of Kw can be used to calculate the concentrations of H+ and OH- ions in a solution. For example, if the pH of a solution is 7, then the concentration of H+ ions and OH- ions are the same. We can use the equation below to solve for the concentration of ions:
Kw = [H+][OH-]
Since the concentration of H+ and OH- ions are equivalent in a neutral solution, and Kw=1.0 × 10-14, we can take the square root of Kw to find that the concentration of each ion is 1.0 × 10-7M.
Note: The ionization constant of water is temperature-dependent, and its value increases with increasing temperature. This is because the rate of the ionization reaction increases with increasing temperature.
The relationship between Ka, Kb, and Kw can also be expressed through the following equation:
Kw = KaKb
Because the range of the pH scale is defined by Kw, we can solve for Ka of an acid or the Kb of its conjugate base if we have the value of one or the other.
pKw is the negative logarithm of Kw. pKa and pKb are the negative logarithms of Ka and Kb. The equations below can be used to calculate the values of Ka and Kb if Kw is known, and visa versa. They can also be used to calculate the pH of a solution if Ka and Kb are known.

Understanding the strength of acids and bases extends beyond the textbook; strong acids and bases can be extremely hazardous if they’re inhaled, ingested, or if they come into contact with our eyes or skin. Learning the fundamentals of acid and base chemistry can help us safely use them in the fields of chemistry, biology, medicine, and more.
