Inflate their knowledge of Gas Laws
Macy’s Thanksgiving Day Parade balloons an annual US holiday tradition, but not all spectators realize that they are also a great example of science and engineering at work. You can use these multi-storied helium-filled cartoon characters as a fun and relatable way to introduce Gas Laws. For example, you could ask, “How many moles of helium are in the Pikachu balloon floating down 6th Avenue?”

To answer this, they would need to know a couple of facts.
- The typical Kermit, Garfield, or Dora the Explorer balloon can contain 12,000 ft3 of helium
- The average temperature in NY City on Thanksgiving morning is about 500F
- The average atmospheric pressure is about 1 atmosphere
To calculate the moles of helium in the balloon, the students will need to understand the relationship between these gas properties. What better way to expand their knowledge than to explore these relationships through some simple experimentation!
They can relate pressure and volume by attaching syringe and tubing to the pressure port on the Chemistry Sensor and collecting pressure data as the volume of air in the syringe changes.
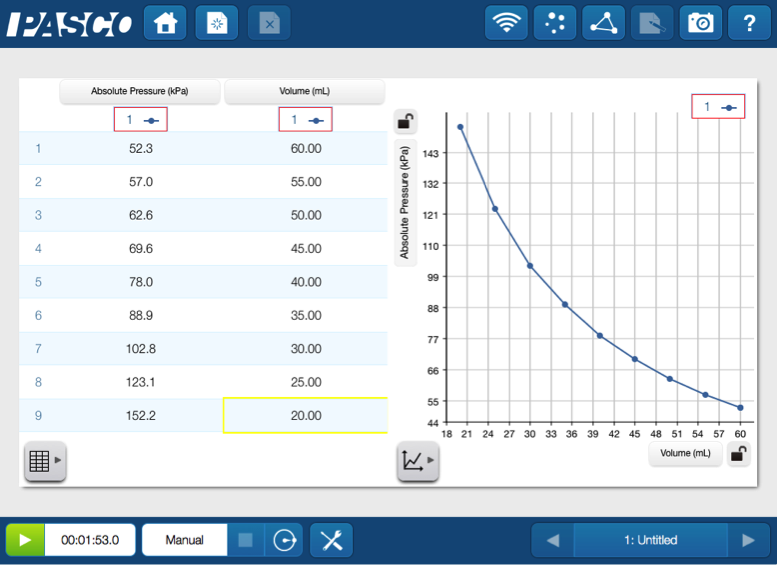
As the data comes in, students can begin to see that pressure and volume are inversely related. This can be mathematically represented as P α 1/V
But, don’t let their egos get overinflated— they still need to incorporate temperature.
To quickly illustrate the pressure and temperature relationship, they can put the tubing into a 1-hole stopper and insert the stopper into a small test tube. Have the students place the test tube and a temperature probe into a hot water bath and plot Pressure vs. Temperature on a graph.
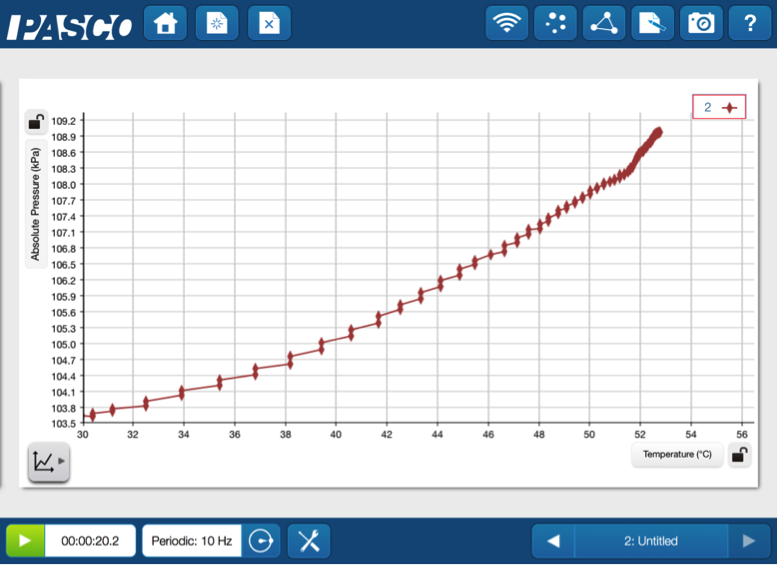
The heat increases both the temperature and the pressure. This is expressed as P α T.
They can construct the explicit relationship between pressure and temperature (in Kelvin) by putting the test tube into hot and cold water baths, letting the measurements equilibrate, and recording the pressure over a wider range of temperatures.
Now the question remains: how are moles incorporated into this parade of variables? The students can model this by measuring the pressure while adding samples of gas to a bottle of air.
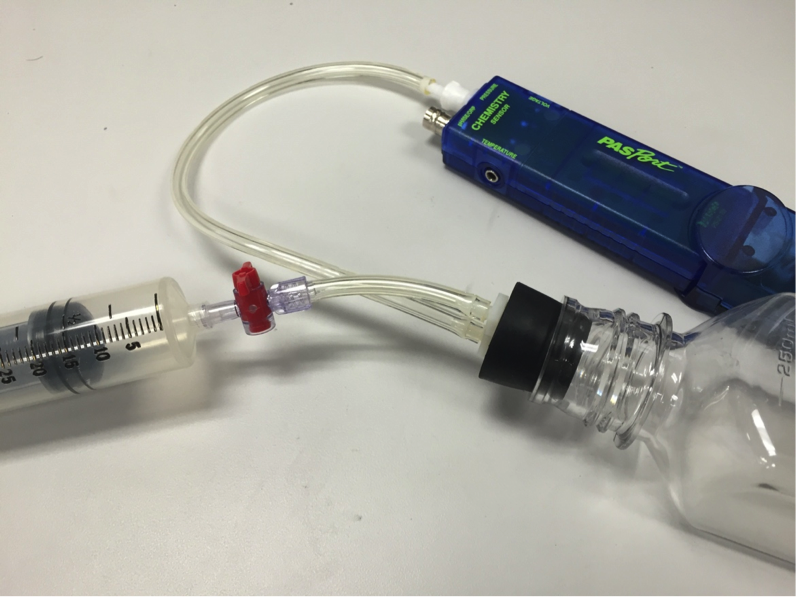
Using a bottle, a two hole-stopper, a syringe and the pressure port on the Chemistry Sensor, the students can quantitatively measure the pressure as more air is added.
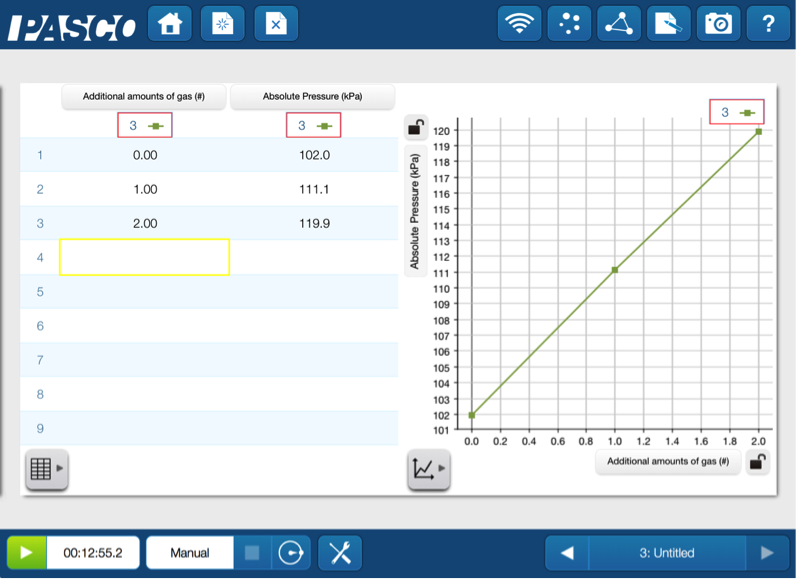
This direct relationship can be expressed as P α n.
Combining the variables, the student can see that P α nT/V.
To get all the variables lined up and marching down their route, this proportionality can be re-written as the equation PV=nRT. Just like Santa Claus is a constant at the parade, the R is a constant in this expression. In this case R = 8.314 L·kPa·mol-1 ·K-1.
Once your students convert the units in this problem to units that match the constant, R, they can finally calculate how many moles of helium it took to keep their favorite float afloat.
For more information about the Thanksgiving Day Parade and the engineering that goes into fabrication of these iconic creations check out the article from Popular Science.
Hope you had a Happy Thanksgiving!



