Help Students ♥ Chemical Changes
With Valentine’s Day quickly approaching, the chemical changes observed most often are due to a little love in the air. Struggling to get your students to focus on real chemical reactions? “Wack-A-Pack” self-inflating balloons can provide some surprising inspiration!

The components of a self-inflating balloon are citric acid, baking soda and water. They are kept separate until the system is “wacked” causing them to mix and react. Student might be familiar with baking soda – sodium bicarbonate – from recipes. They also might know that citric acid gives citrus fruits their sour taste. Solid citric acid can be found in some food stores and online. Though it is used to give sour candies their sour taste – but don’t let them taste it during the experiment! We believe in a strict “don’t eat the experiment” policy.
Start the experiment with a microscale version of the reaction to hone the students’ observational skills. Have them put a drop of water on a clean experiment area. Then add a pinch of solid baking soda and a pinch of citric acid. When both solids dissolve in the water, something surprising happens—science is in the air! Bubbles form and there is an audible fizz as gas forms. Evidence of a chemical reaction!

Of course, they will want a bigger reaction. Take their enthusiasm and turn it into a learning opportunity and engineering project. Challenge them to create a device to capture all the gas that is formed. One simple example could be to put the solids in a sealable bag then put the water in a small cup (so it cannot mix) into the bag and seal it up. For an extra challenge, see if they can come up with a ratio of reactants that inflates the bag without popping it!

After it is sealed, give it a quick shake and see what happens. No need to buy a Wack-A-Pack for Valentine’s Day because they’ve just made their own self-inflating balloons!

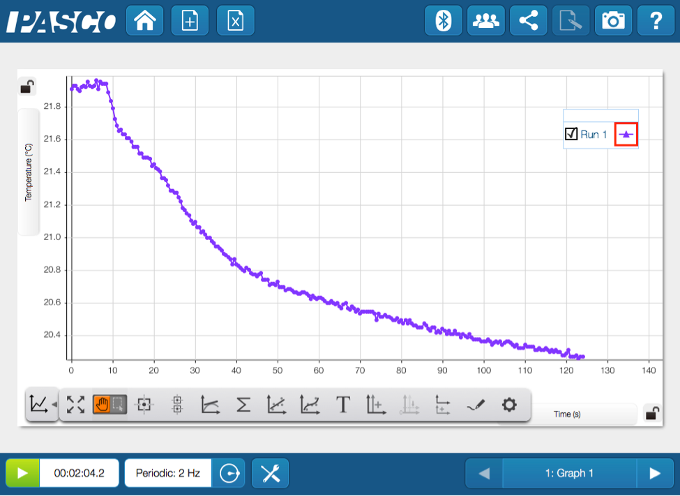
Your more observant students will notice that the bag started to feel cold as they were shaking it. Was it just their imagination or did the temperature really change? Here is an opportunity to gather some more evidence! This time, perform the experiment in a cup with a Wireless Temperature Sensor. Put the sensor in the water, start collecting data, then add the solids.

What does the data indicate? It was not their imagination - the temperature definitely drops as this endothermic reaction occurs.
Make observations of a chemical reaction meaningful, engaging and fun, with some household items and inspiration from a ”Wack-a-Pack.” It is an experiment that they will truly ♥.


