An Amped up Electricity Lab
Looking for a fun way to to spark your students interest in electrochemistry while they continue to build their engineering skills? Let them engineer a battery to light up an LED! With some simple materials, they can wind their way through a circuit of activities to reach the desired learning outcomes.
To get started all they need are some different metal samples and an electrolyte solution (in this activity citric acid from lemons). To measure the voltage of the battery I used a Wireless Voltage Sensor, some leads with alligator clips, and SPARKvue software.

The first in this series of step toward battery production is to find the best combination of metals. Copper, zinc, iron, nickel, and lead were essential elements in our experiment. Just put two slits in the lemon peel and measure the voltage of different metal combinations.
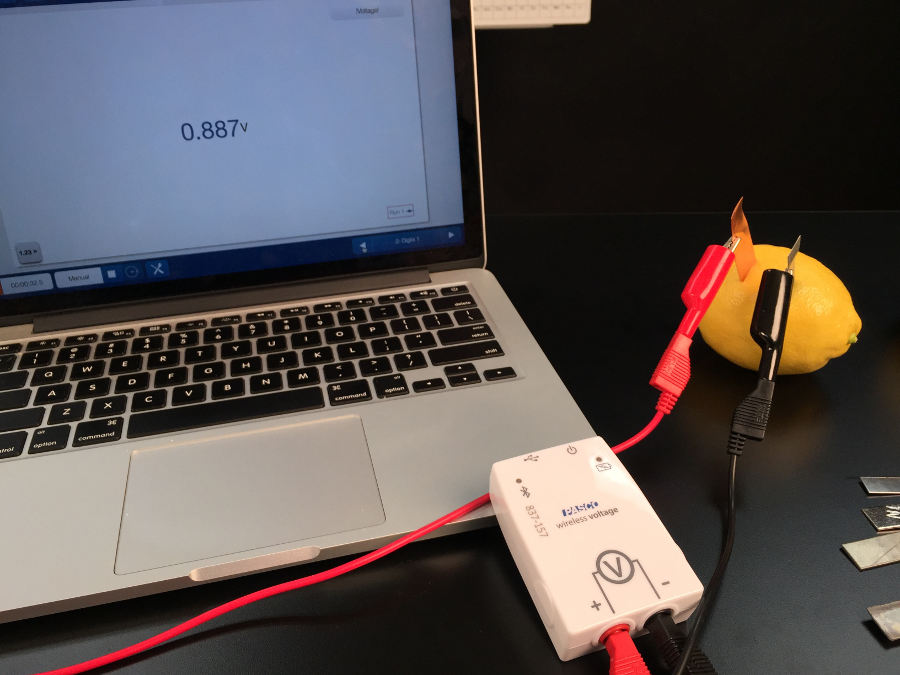
As they explore, students will observe that different combination of metals have the potential to produce different voltages. At this point, you have the opportunity to address the misconception that the electricity is generated from the fruit or vegetable (I blame the potato clock kits). The electricity is not coming from the lemon, but is actually generated from differences in the metals’ ability to gain or lose electrons. They can even prove it’s true when the pair of metal strips are the same element (for example copper with copper) and the voltage reads zero.
If they get a negative voltage reading, instruct them to switch the red and the black leads. Now you have to opportunity to introduce cathodes and anodes and the rational for the (+) and (-) symbols on their batteries at home.
Once they find a good combination of metals, they can test the LED— but the little bulb will not light. What could they do to get more “juice” to the bulb?
Your students might remember that household batteries in electronics are typically stacked together. This increases the voltage. They can do the same thing with their lemon batteries. Using some addition wires they can put the metal combinations together to form a series of batteries. So if the lab supply closet gives you lemons, make lemonade batteries!
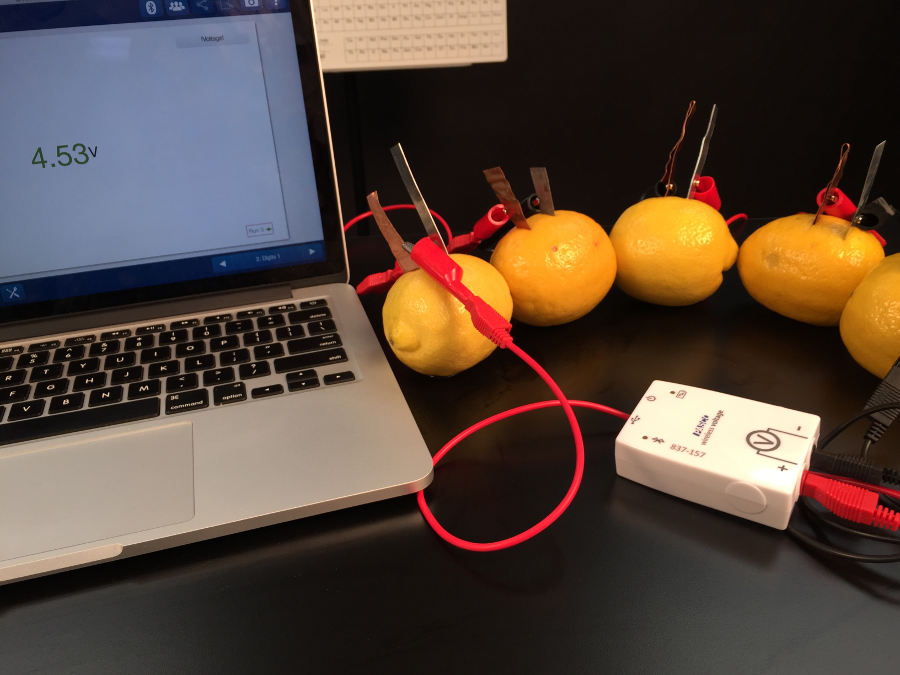
With a series of 5 batteries of the same combination of metals, the voltage is now about five times greater that it was for a single battery! Finally, it’s time to light that LED.

This engaging activity is a fun way to introduce fundamental concepts while asking students to think like engineers. By testing the different combinations of metals, discovering which way the current in their makeshift battery flows and discovering how many cells it takes to reach the voltage necessary to light the LED they not only gain a practical understanding of how batteries work but learn to collaboratively test and revise their setup to solve a problem. After this exercise you will now have greater potential to dive deeper into electrochemistry topics like oxidation, reduction and electromotive force.
Note: Wireless sensors like the Wireless Voltage shown here use Bluetooth® Smart technology and connect directly to your devices. Windows computers, Chromebooks and older Macs require a low cost USB Bluetooth radio to use them. See pasco.com/compatibility for full details.
Free Downloads:
Here are a handout and PowerPoint presentation that have been used when performing this activity as part of a PASCO workshop.


