Dissolved Oxygen Inquiry
Dissolved Oxygen (DO) is simply oxygen gas (O2) dissolved in water, but it’s one of the best indicators of a healthy aquatic ecosystem. For most freshwater environments, a DO concentration of 5–10 mg/L (or ppm) is needed to sustain fish species. Many factors can affect DO concentrations and the solubility of oxygen in water. The activity below examines some of these factors and presents a great opportunity for inquiry experiments that integrate other disciplines (biology, chemistry, and even physics!).
Demo Materials:
- Data collection system
- Dissolved Oxygen Sensor
- Temperature probe*
- Beakers (3), 250-mL
- Magnetic Stirrer
- Ice water, 200 mL
- Room temperature water, 200 mL
- Warm water (30-35°C), 200 mL
- Wash bottle
*If using a Dissolved Oxygen Sensor instead of a Water Quality Sensor, use the Fast Response Temperature Probe to prevent electrical interference. Do not use a Dissolved Oxygen Sensor with a Stainless Steel Temperature Probe. The Water Quality Sensor can be used with either temperature probe.
Demo Instructions:
To introduce the sensor and lab activity, conduct the following as a demo. Before beginning, discuss the concept of dissolved oxygen.
- Talk about how dissolved oxygen gets into and out of the water in an aquatic ecosystem. Ask students to predict how they think the concentration of DO will be affected by temperature changes.
- Ask about the effects of pressure on the concentration of DO. A 2-L bottle of soda is a great analogy to use. Ask students why soda goes flat if you leave it in a warm place with the cap off.
- Have students brainstorm or research other factors that can affect DO.
Procedure:
- Before starting, warm up and calibrate the Dissolved Oxygen Sensor (video link) in room temperature water. Calibration is not critical to the demo but recommended.
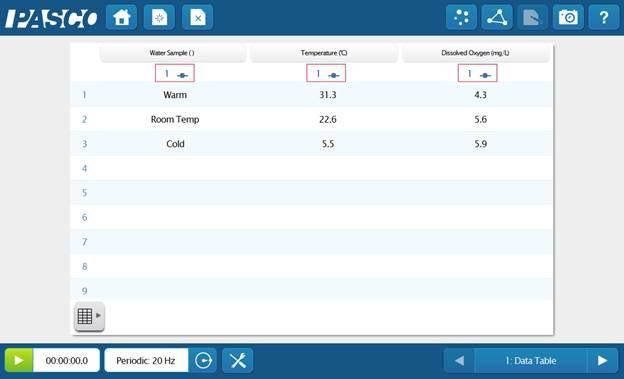
- Create a table of DO and temperature, and change the sampling mode to manual.
- Place the sensor in the warm water on a stir plate and begin monitoring data.
- Insert the probe, wait for the measurement to stabilize, and save the data point.
- Repeat the procedure with the room temperature water and cold water. (Remove any ice from the cold water before sampling.)
- Rinse the Dissolved Oxygen Sensor and return it to the storage bottle.
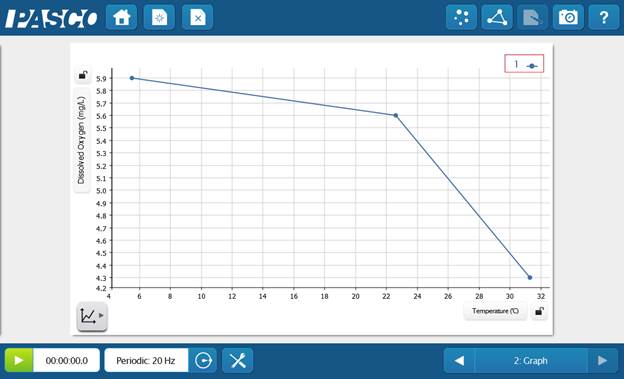
- Create a new page with a graph of DO concentration vs. temperature.
Inquiry
Now that students have seen how to use the sensor and discussed factors that can affect DO concentrations, they can design and conduct their own experiments. Many relevant variables are easily manipulated in the lab environment. Using other PASPORT sensors, students can easily quantify the dependent variable as well. Here are some suggested variables for students to consider:
- Temperature
- Atmospheric pressure
- Presence of photosynthetic algae or plants
- Presence of decomposition
- Flow rate
- Wind
- Pollutants such as nitrates, sodium sulfite, phosphates, or ammonia.
Other Resources:
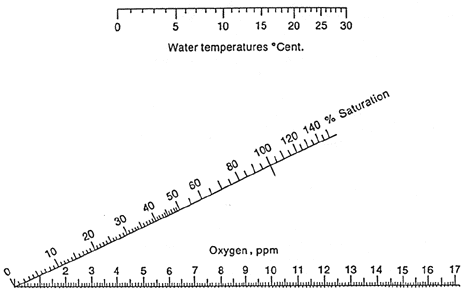
This nomogram can be used to calculate the % saturation from water samples if the temperature and oxygen concentration are known. Use a ruler or other straight edge to line up the water temperature and oxygen concentration. The straight edge will intersect the % saturation scale at the estimated value.