Working for a Winter Wonderland
Instant snow is a fun substance for kids of all ages and a great tool for engaging young scientists in physical science concepts related to matter and its interactions. And the new Wireless Temperature Sensor helps to make it a quantitative one as well!
While many of you may have snow right out side your classroom door, scientists never take that easy way out. The first step to creating our indoor winter wonderland is to gather the materials. Instant snow is a polymer called sodium polyacrylate that expands as it absorbs moisture. By collecting and analyzing data as they perform the experiment you will be incorporating science and engineering practices into the lesson and challenging your students to answer the question “Is it a physical or chemical change?”

I used about 12 grams of instant snow, 160 mL of water and a Wireless Temperature Sensor with SPARKvue to analyze the data.
After starting data collection, I added the powder to the water and let it snow, let it snow, let it snow.

By checking the total mass before and after the water is added, students can see that the mass is conserved even though the volume is definitely not.
The temperature data will help your students crystallize additional insights about the experiment.
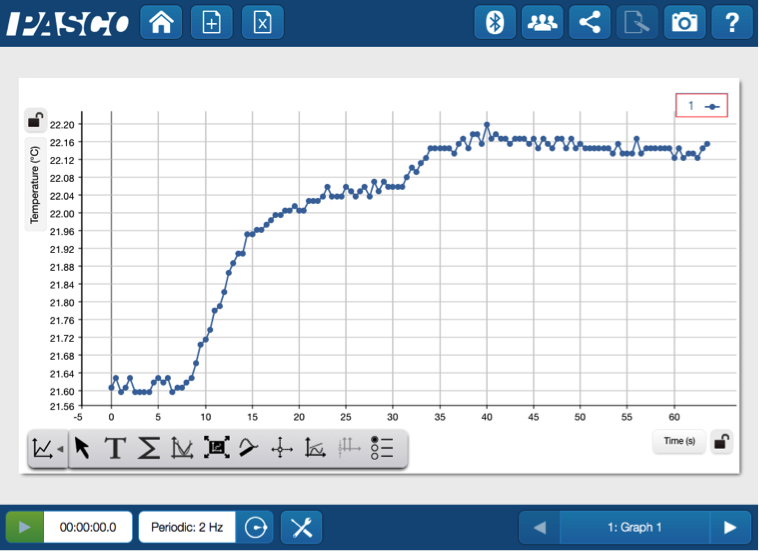
For example, students will see the slight uptick in temperature as the dry powder absorbs the water. What causes this? This is a great opportunity to introduce exothermic and endothermic processes.
After the initial blizzard of reactions, your students can finally dig into the snow. The best part— no winter gloves or scarves needed! They might notice that the snow is cool to the touch. This experiment provides an avalanche of opportunities for temperature data exploration.

Simply remove the temperature probe from the pile of snow, and begin a new run of data.
It’s not nearly as cold as the white stuff outside, but there is a noticeable drop in temperature.
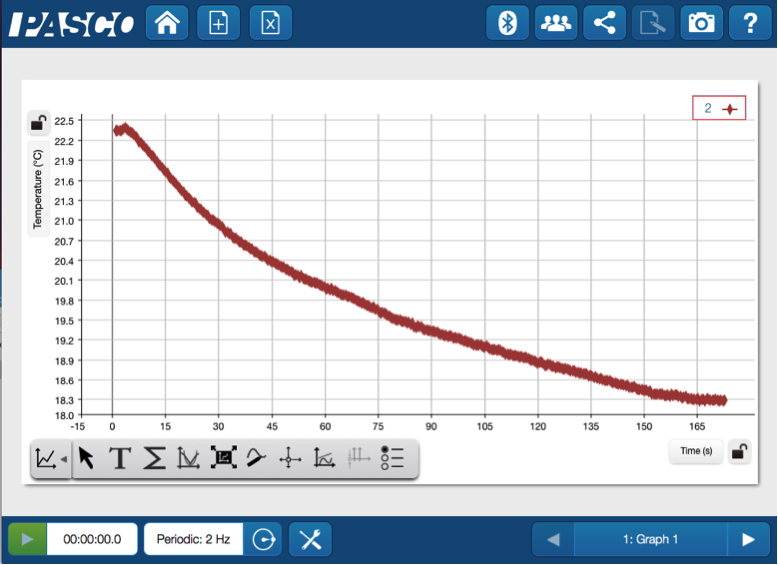
With their observations, the mass data and the temperature data, students can snowball the evidence that they have gathered to answer answer the initial question about whether this is a physical or chemical change. You could even split class into two groups and have them fight it out to determine what type of change it is— though I’d recommend you have them use their data as evidence for the battle instead of snowballs.
Now it’s time to for the students to unleash a flurry of ideas that could extend be used to this activity. Some questions could include:
- What is the best ratio of instant snow to water?
- Can I get the original dry powder back?
- What would happen if the water had food coloring in it?
- What does a model of a molecule of sodium polyacrylate look like?
And when the experiment is complete and you have a giant pile of snow, you will probably also be asked, “Do you want to build a snowman?” To which the only appropriate response is “Let it go.”


