Watershed Studies: Oh-no! Where's my DO?
Earth disturbances are fairly common in areas with construction or mining, but what exactly is the impact of this type of disturbance? And how can you show your students about the chemical changes that are taking place and the resulting impact on the watershed?
Here’s a quick demo to model the impact of Acid Mine Drainage (AMD) which occurs as a result of water filtering through rock that has high concentrations of sulfide minerals. The most common of these minerals is iron disulfide (FeS2), commonly known as Pyrite or “Fools Gold”. Pyrite is concentrated in waste rock from mining activity and, when exposed to water and oxygen, the resulting reaction forms sulfuric acid (H2SO4).
Here are some of the reactions that are occurring:
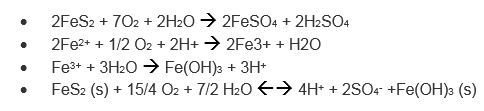
To model this process in the lab, gather the following materials:
- Advanced Water Quality Sensor with pH and DO probes
- 500mL beaker
- 400mL of tap water or a water sample from a local body of water
- ~2-6g of pyrite— either crushed or dust
- Stir Plate (optional)
- SPARKvue for data collection and analysis
Will the DO and pH change, by how much and how fast? Why? Use the prediction tool in SPARKvue or ask students to write a hypothesis for the experiment.
Calibrate and then set up the sensors. Conduct a short run (30-60 seconds) with the sensors in the sample to establish a baseline, then slowly add the pyrite sample to the water while stirring slightly to mix. Allow the data collection to proceed until the reaction has stabilized, usually ~10-15minutes.

Ask students to make some observations and answer a few questions as the reaction proceeds. Here are some suggestions to get them started:
- Are there any noticeable color changes to the solution?
- What, if any, trends are immediately visible in the data?
- What are the limitations of this model compared the weathering happening in a real watershed?
- Based on what you’ve observed so far, what impact might this process have on plants and fish? And what impact would it have on weathering of other minerals? Or communities that use lakes/streams for a source of drinking water?
- What could be done to mitigate this type of weathering when earth is disturbed for mining or construction?
After the reaction slows/stabilizes, stop data collection and ask students to determine the change in pH and DO.

Although other minerals may help neutralize the acid, this reaction (if unmanaged) can damage watersheds and result in visible changes to water chemistry. In extreme situations this can stain rocks yellow or orange, often called “Yellow Boy” when ferrous oxide (Fe(OH)3) precipitates. While students can’t save a watershed through a simple lab, they can learn valuable lessons that will prepare them to become responsible environmental stewards as they become active global citizens.

Recommended Sources;
“Acid Mine Drainage: Chemistry”. Classroom of the Future (COTF), Nov. 2004. Web. 20 Oct. 2015.
Weiner, Eugene R. Applications of Environmental Chemistry: A Practical Guide for Environmental Professionals. Boca Raton: Lewis Pub., 2000. 156-57. Print.


