Under Pressure - Wireless Sensor Inside a Balloon!
When introducing pressure and gas laws, I have always used balloons as a conceptual starting point because they are a familiar item to students. Unfortunately, I could not easily measure the actual pressure inside of the balloon— until now. The Wireless Pressure Sensor not only makes it possible, but very easy to do. Pretty amazing right? What’s next, life on mars?

Even for those who are absolute beginners to pressure studies, this is a fun experiment that’s sure to garner you some "cool points" from your students. Using a slightly larger balloon, and a little patience, students should have little difficulty fitting the pressure sensor inside of the balloon.
Now, with the sensor secured inside the balloon, it's time to increase the pressure! Turn the sensor on, and connect it to your device. Commence a countdown, and start collecting data as students inflate the balloon.

As the environment within and around the balloon changes, students can use the analysis tools within SPARKvue to observe the changing pressure in real time.
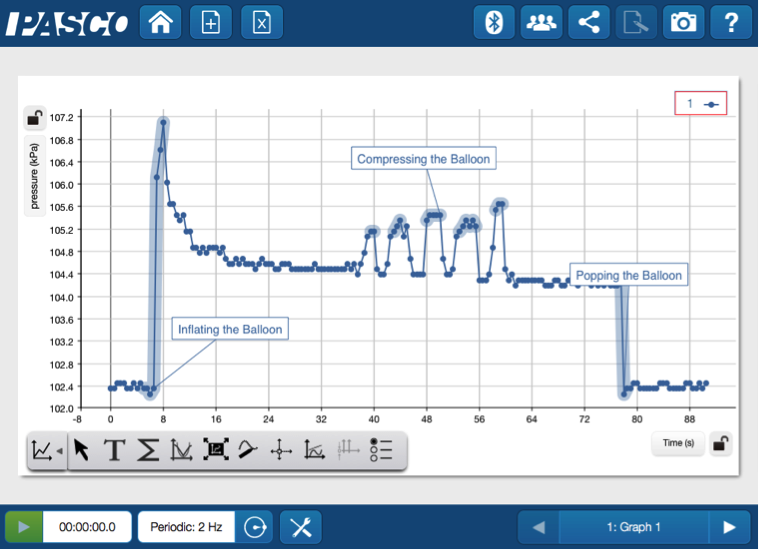
Some things to initially note include: the slight increase in pressure as the balloon is inflated, the changes in pressure as the balloon is compressed, and the sharp decrease in pressure when the balloon is popped. This simple experiment provides an incredible exploration of the many phases of pressure changes. Seeing data displayed in real time, alongside the balloon's visually observable changes, helps students connect the science behind the sight and sound of a balloon popping.
Now that they have engaged in the activities and explored the data, try having students explain and elaborate on what is happening and why.
This activity only takes a few minutes to complete, making it an excellent demo or an engaging investigation. It also works well when grouped with other basic investigations that enable students to move station to station. As students begin to construct their knowledge about gas properties using familiar items, you can incorporate the empirical gas laws leading up to the ideal gas law.


