Physical And Chemical Changes
You want an argument, bring some evidence!
What do students look for when they are trying to distinguish a physical change from a chemical change? Sure, things like a change in temperature, color, or the formation of bubbles are typical signs of a chemical change, but often those indicators are not enough. What other evidence can students site as they try to determine if a reaction has occurred?
To move beyond qualitative observations, they can collect data on the substances before and after they are combined. In this activity, students are given four beakers. In addition to visual observations, with the additional of sensors they can collect pH, temperature and conductivity data to analyze and interpret.

The first pair of beakers contain a blue and a yellow solution. Using the Wireless pH, Conductivity and Temperature Sensors, students can collect the initial data.
| Beaker | Color | pH | Conductivity (mS/cm) | Temperature (oC) |
|---|---|---|---|---|
| 1 | Blue | 6.37 | 48.3 | 22.1 |
| 2 | Yellow | 6.13 | 43.7 | 22.0 |
When the blue and yellow mixtures are combined, a green color is produced.

Was this simply a case of “yellow and blue make green”, or did a chemical reaction occur? To answer this question with evidence students need to collect and interpret data!
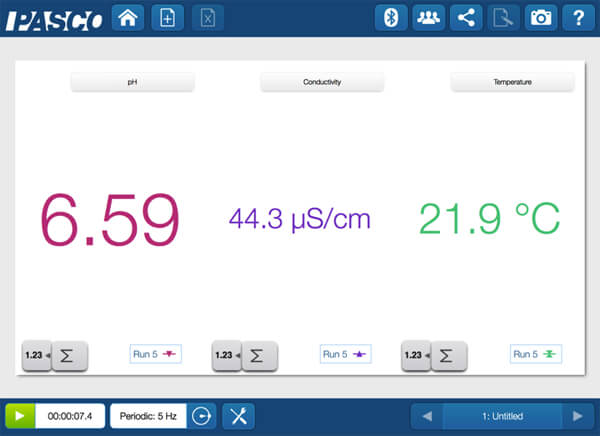
The color changed, but the measurements essentially stayed constant. Was a physical change or a chemical change? Your students have the evidence– let the arguments begin!
Now they can explore the solutions in the other beakers. Again, the students should make initial observations and collect the data.
| Beaker | Color | pH | Conductivity (mS/cm) | Temperature (oC) |
|---|---|---|---|---|
| 3 | Clear | 1.84 | 20,938 | 21.7 |
| 4 | Clear | 11.99 | 19,928 | 21.7 |
When the two clear solutions are combined, it looks like nothing happens.

There are no bubbles, and no color change. But does this mean nothing changed? For more definitive proof they need more evidence. Again, it is time to collect, analyze and interpret the data!
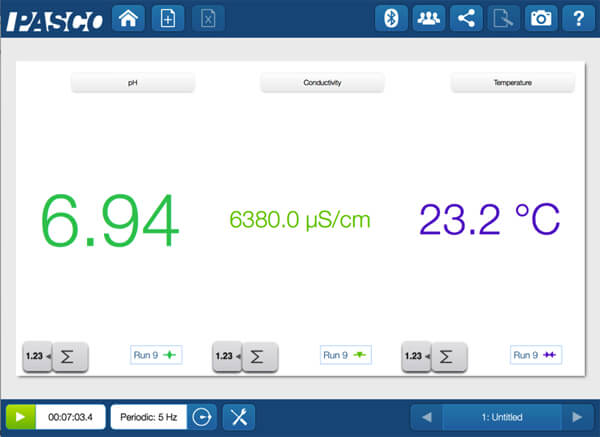
The pH neutralized, the conductivity dropped, and the temperature increased. So, is a physical change or a chemical change? Round two— let the arguments continue!
Your students will find that some processes are hard to classify as simply a physical or a chemical change. There are some typical chemical change indicators that they should know, but they are just indicators, not proof. Throughout this activity with some ambiguous processes, students can sharpen their science and engineering practices. By looking for patterns and analyzing and interpreting data, they will have a chance to argue based on the evidence!
Did you know that PASCO now has a complete chemistry curriculum? Essential Chemistry features an innovative eBook, corresponding hardcover textbook, lab manual and innovative PASCO equipment.




