Mole Day
It’s that time of year again. Chemistry teachers everywhere are dusting of their pun-ny jokes and creating mole-themed, activities and treats to celebrate National Mole Day in commemoration of Avogadro’s number (6.02 x 1023). Mole Day is celebrated starting at 6:02 am on October 23rd. How will we be celebrating? With chips and Guaca-mole, of course!

As we introduce the topic to students we typically start with something they already know. A mole, like a dozen, is a way of counting things. It just so happens that a mole is a really large number. While 1 dozen equals 12, 1 mole equals 6.02 x 1023. This number has to be very large because the particles that we deal with in chemistry happen to be very, very small (or sm-ole… the bad jokes keep on coming).
To give students a mole-ecular perspective of those tiny particles, you can use a modeling kit.
At this point in the year it is important for students to understand the basics of chemical formulas. The model kit gives them something to see and touch as they learn that subscripts in a formula represent the number of atoms that are bonded in the compound.

In addition to formulas, the model kit can be useful in calculating molar mass. Since each different colored atom represents a different molar mass, students can just take their model and add up all the masses of all the atoms that they see! In the case of water, the oxygen is 16 grams per mole and each hydrogen is 1 gram per mole. In total, there are 18 grams for every one mole of water molecules.
So how do chemists relate these tiny particles to something that they can measure, like grams? Moles to the rescue! Avogadro’s number (the number of particles in one mole) and molar mass (the amount of grams in one mole) both meet in the middle at … moles.
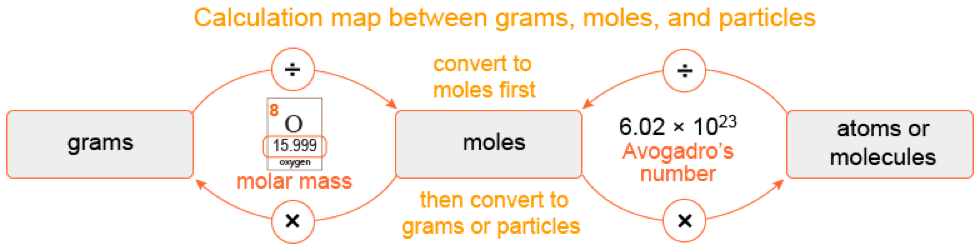
Moles are central to counting particles in chemistry. Using Avogadro’s number and the molar mass of water, we know that there are 6.02 x 1023 molecules in graduated cylinder that contains 18 grams of water.

Model kits can be great tools in helping students visually and kinesthetically learn about chemical formulas. They become actively engaged in the learning process as they discover the meaning and value of subscripts, molar masses and, of course, Avogadro’s number. And as students gain a better understanding of these concepts the real fun begins— they start to really understand your science humor! “Oh no! I’ve spilled water on my book!”

Happy Mole Day!

