Modeling Conservation and Change
Baking soda and vinegar create a classic chemical reaction. Bubbling volcanoes and gushing fountains made with simple household items— crowd favorites. Everyone loves it!
It is also a great reaction to address some core physical science standards. Students can make observations before and after— and they can easily see that a chemical change has taken place. With a some extra material, you can also use this reaction to address other core concepts like the conservation of mass and the rearrangement of atoms.
To do this, we will first gather some supplies. We’ll start with the vinegar which contains the chemical acetic acid (CH3COOH). Instead of baking soda, we will use another substance that also contains sodium bicarbonate but is much easier to distribute evenly to lab groups— antacid tablets (NaHCO3). And to address the conservation of mass we need a balance. Finally, and a Molecular Model Kit can be used to help students visualize what those substance look like.

Next up, we need a reaction vessel that will expand to contain the gas that is produced. Ask you students for ideas, and in very short order they will likely suggest a balloon. Pour some vinegar into the bottle and add a small piece of the antacid table to the balloon. Next comes the tricky part! Attach the balloon to the bottle without letting any part of the tablet fall into the vinegar.

Now that everything is set, we need to determine the mass before the reaction happens. We can do this by putting the entire reaction vessel on a balance.

Stop! Reaction time! Simply lift the balloon and observe as the reaction begins.

Notice that the mass stayed the same, but the balloon filled with gas. How could this be? To answer that question, let’s look back at the models.
Acetic acid (CH3COOH) and sodium bicarbonate (NaHCO3) are the reactants.
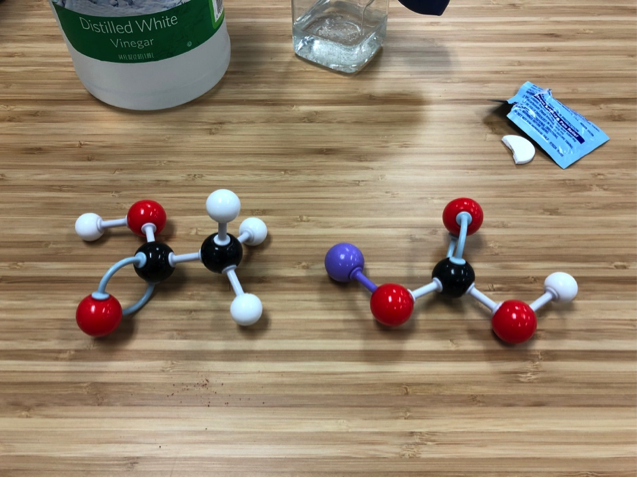
Those reactants turned into sodium acetate (NaCH3COO) with water (H2O) and, of course, the carbon dioxide (CO2) that filled the balloon.

Using the models we can see that all the atoms are still there, so the mass is conserved. But they were rearranged so the products have different properties! Using physical models, like a model kit, helps students develop the mental models that are needed to construct their own understandings.


