Light(saber) Emission Spectroscopy
Lightsabers are not only the weapons of choice of Jedi Knights and those that have fallen to the dark side of the Force, they can also be great tools to introduce emission spectroscopy and atomic theory to the Padawans in your chemistry class.
They come in a variety of colors— red, green, blue and even purple (if you are cool enough to be Mace Windu). What does the color of the light imply about the energy of the blade, and what could this possibly have to do with atoms?
You don’t think we can study it? I find your lack of faith disturbing. Luckily, we can use the PASCO Wireless Spectrometer, Fiber Optic Cable and Spectrometry software to look at what makes these sabers into lightsabers.

First we looked at the Jedi blades of choice – green and blue – by simply pointing the end of the Fiber Optic Cable at the lightsaber’s light source.
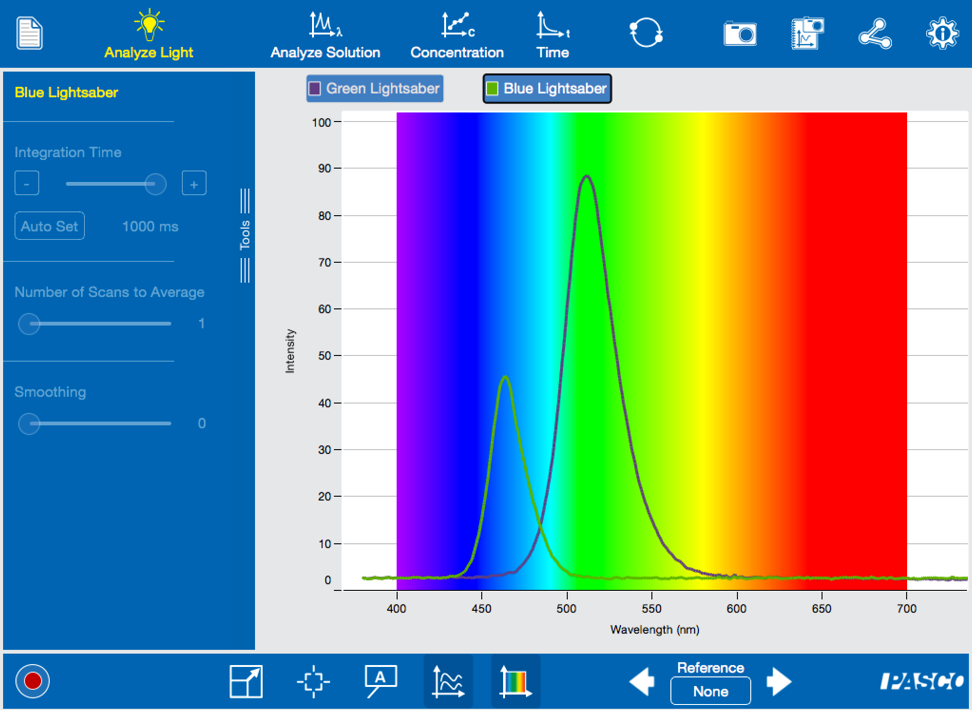
Sadly, these aren’t the elegant weapons of a more civilized age, they are only toy lightsabers. These particular toys use LEDs that are covered by a plastic blade. The light sources have a narrow spectrum with a wavelength that has a maximum intensity around the color that you see.
Based on the intensity peaks in the spectrum it looks like the green blade is the better weapon. But, if the laws of physics were true even a long time ago in a galaxy far, far away, the inverse relationship of wavelength and energy (E = hc/?) indicates that the blue blade would have a higher energy than a green blade.
Those that fell to the dark side of the force have chosen a red blade for their lightsaber. We can look at the spectrum to see what powers those blades, as well.
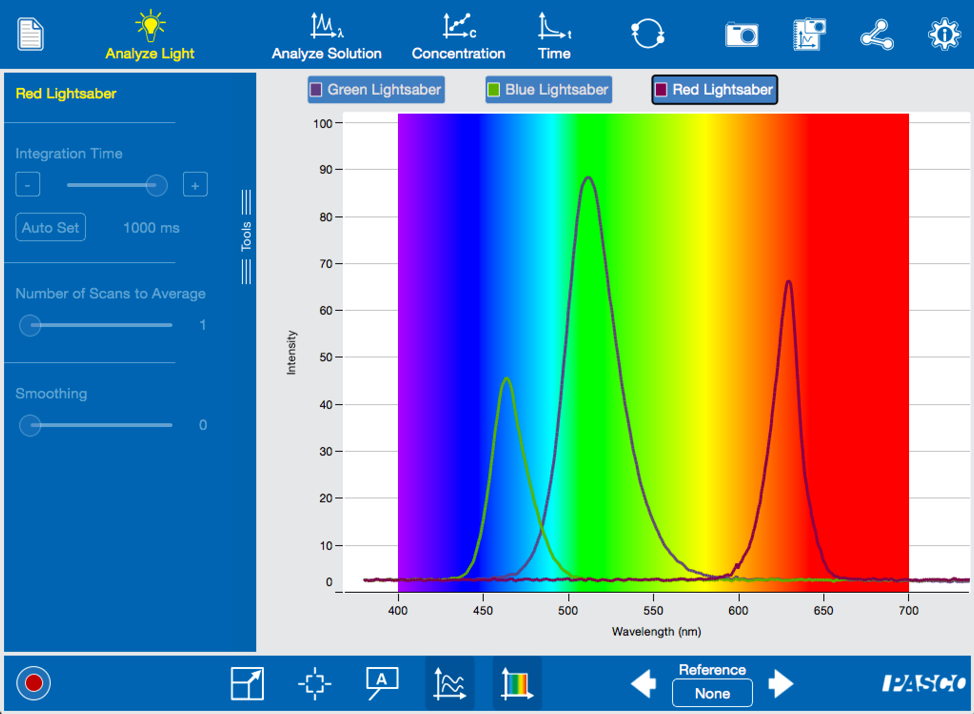
Darth Vader, Darth Maul and Kylo Ren’s red energy blades, have a much higher wavelength than the blue and green blades, indicating a much lower energy. Not only are the Sith blinded by hate, they also didn’t take science into account as they choose their weapons.
Your students are beginning to understand lights, but their training is not yet complete. We still need to connect light emission to atomic theory.
We can use the Spectrometer and Fiber Optic Cable to look at the Emission of a hydrogen gas tube.
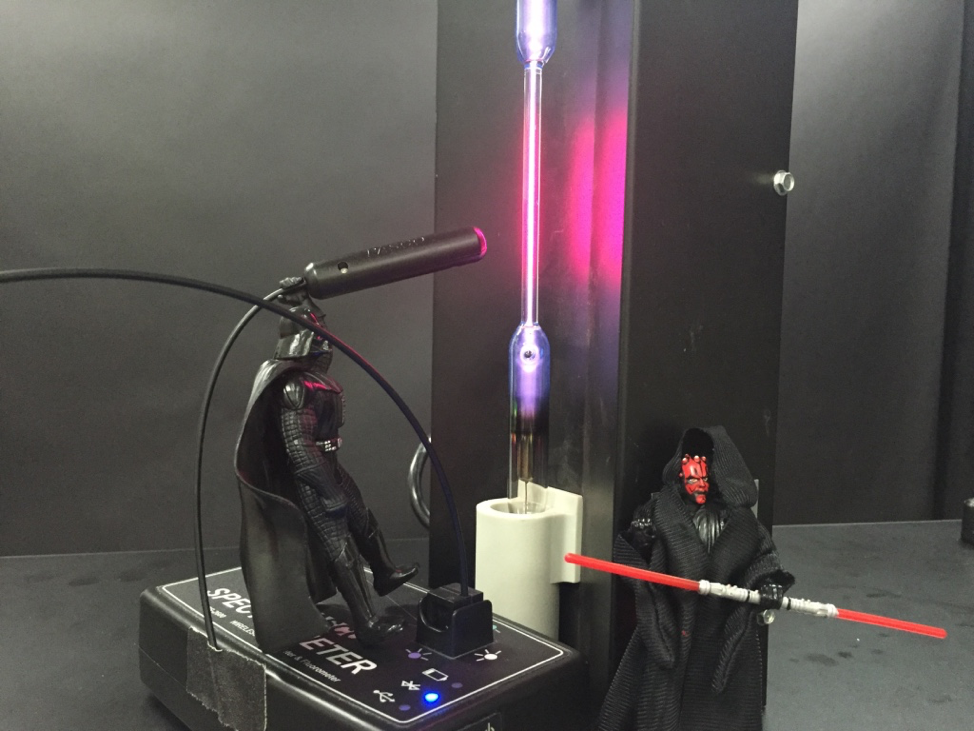
Mace Windu would appreciate the purplish color, but we want the students to collect data and analyze the spectrum.
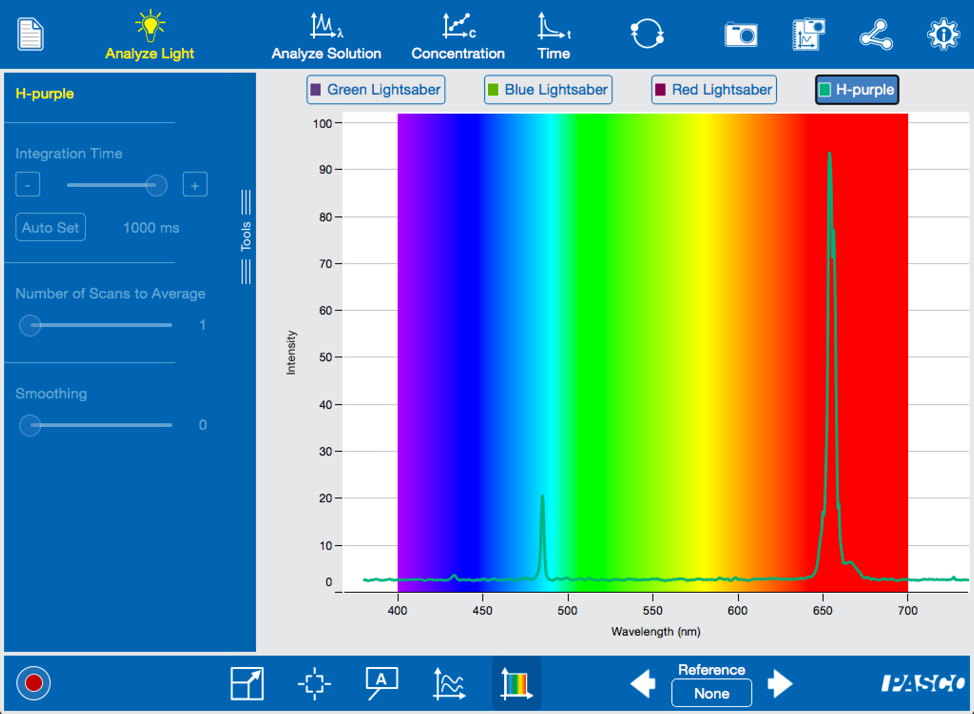
Notice that the purple color that your eyes see is actually the result of few distinct peaks of emission from the hydrogen atom. Like unlocking the blueprints to the Death Star, your students are unlocking the secrets of atomic structure.
Each of the wavelengths represents an electronic transition from higher to lower energy levels. With the tools in the Spectrometry software, your students can determine the wavelength of each peak. They can then calculate the energy of each transition. Just as Luke constructed his own lightsaber, your students are have constructed their knowledge of atomic theory.
If Yoda were talking about science instead of the dark side, I think it would go something like this, “Questions lead to experiments. Experiments lead to analysis. Analysis leads to learning.”


