Introduce Atomic Theory with Light!
How do you make the development of the Atomic Theory more accessible to students? Start by looking at lights using PASCO’s Wireless Spectrometer! To begin the activity, have the students use the Spectrometer to analyze the spectrum of a “white” light source.
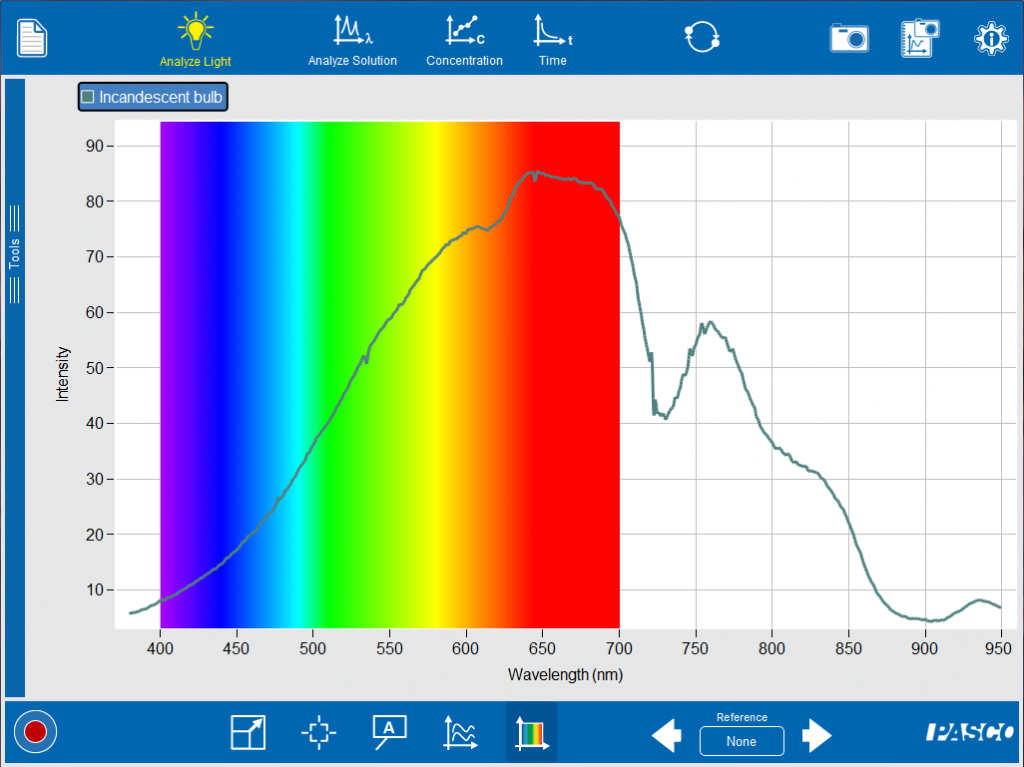
Facilitate discussion by asking, “What do you notice about the spectrum? Is it broad or narrow? Why do you think it appears white?” Next introduce your students to colored lights with LEDs.
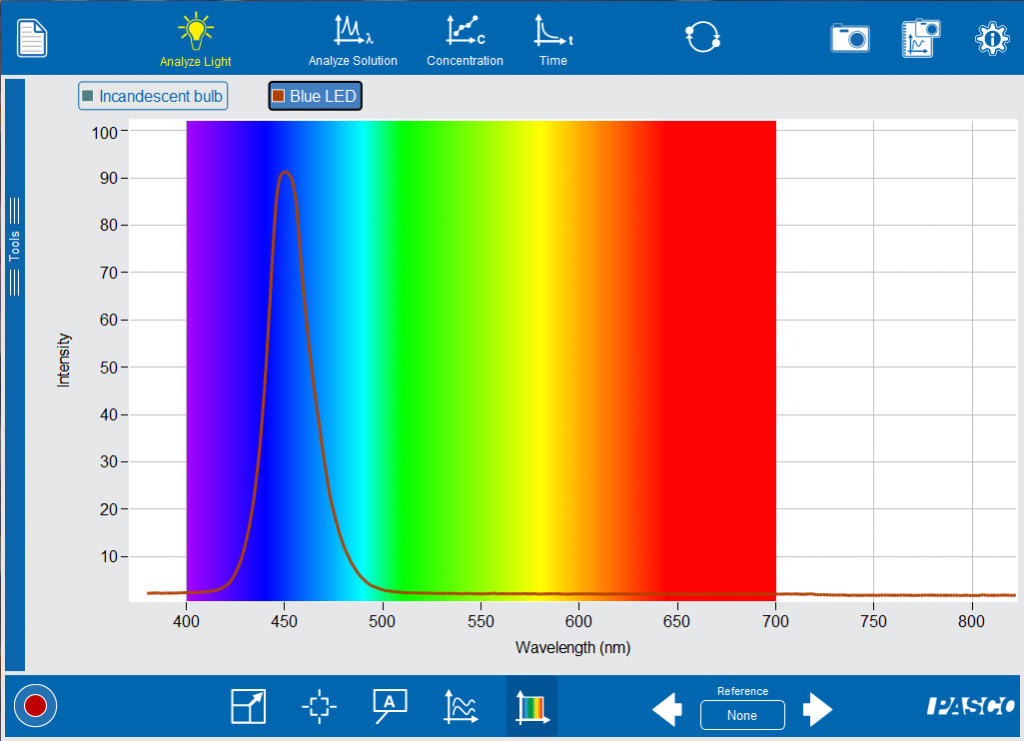
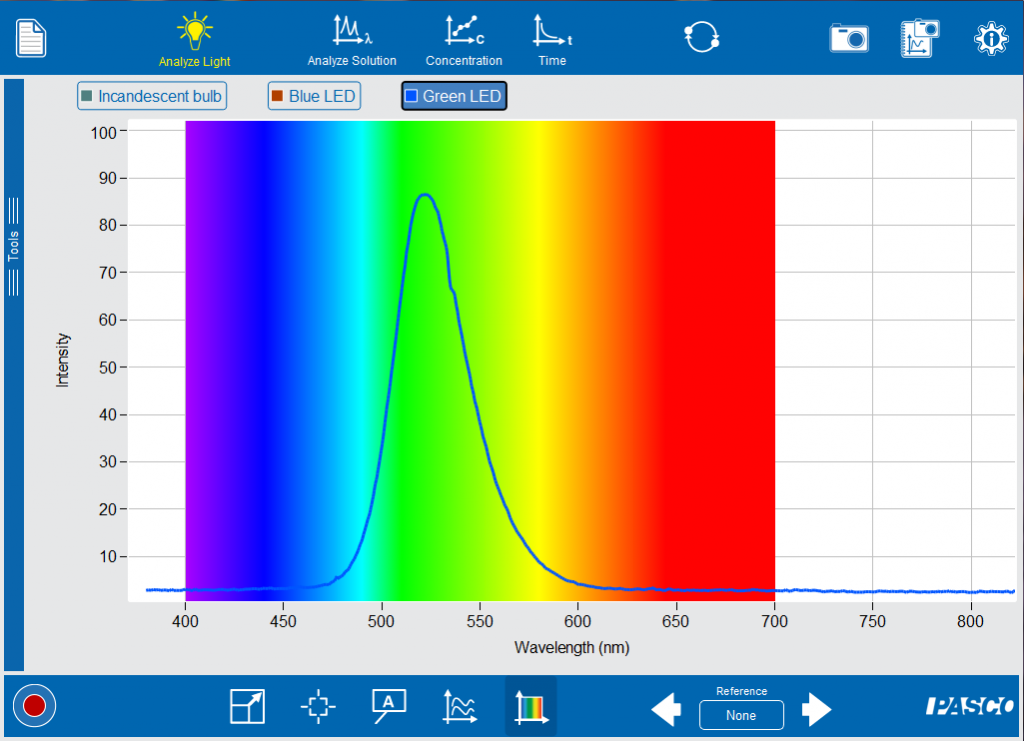
Ask your students to compare and contrast the blue LED, the green LED and the incandescent bulb. Notice that the LEDs have a narrower spectra with a peak intensity that has a wavelength representing the visible color. Now you can start making the connection between the light that is emitted and the structure of the atom. Like Niels Bohr, it is easiest to start with the simplest atom, Hydrogen.

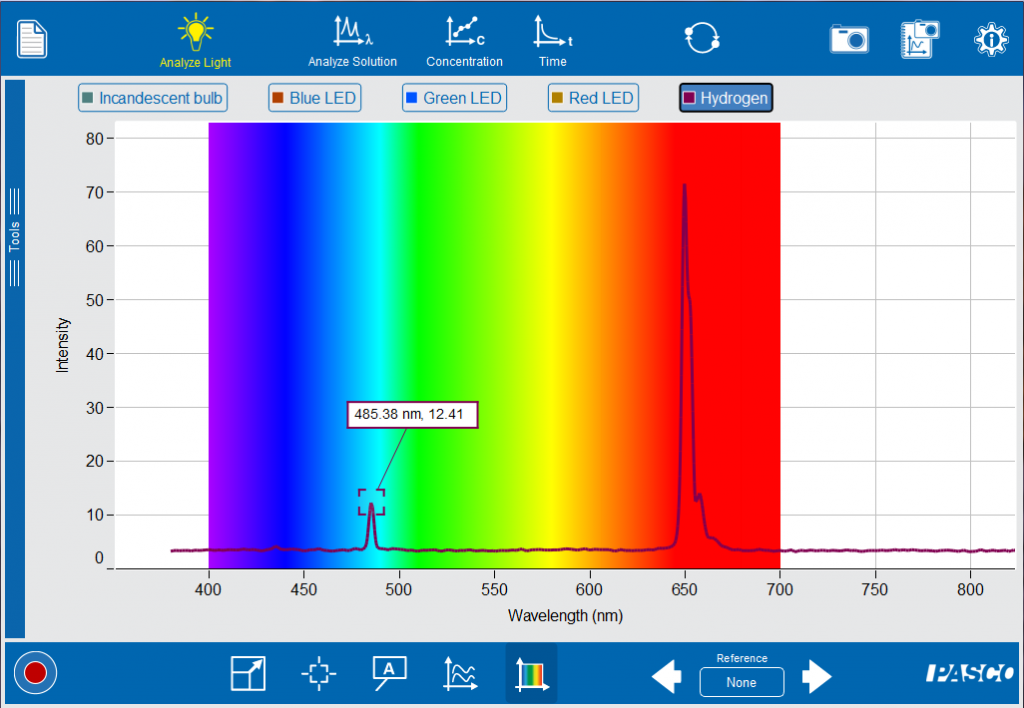
Visibly, the emission looks like a single color. But, when it is analyzed with the Spectrometer, your students will notice that the spectra is actually a collection of very narrow peaks. The peaks represents the amount of energy changed as an excited electron transitions back to a lower or ground state. Bohr eventually develop atomic theory based on the energy of these electron transitions.
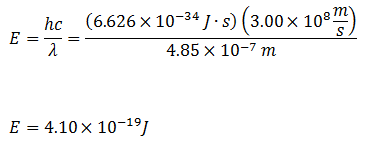
Traditionally, atomic spectra and atomic theory are introduced with very inexpensive spectroscopes or diffraction grating glasses. While it can be fun for student to view the rainbow of a spectrum, it is very difficult to observe distinct emission wavelength values. Using the PASCO Spectrometer, students can be guided through an inquiry activity, starting with known light sources and progressing to quantified emission spectral values. These measured wavelength values can then be use to construct the connection between light and atomic theory.


