Evaporative Cooling
This activity can help refresh your students on the scientific method and provide some biological context to the "chemistry of life" content that often kicks off the school year. Perspiration is critical to human evolutionary history and the endurance it enables is arguably on par with cognitive abilities as Homo sapien superpower.
This quick activity reminds students about the biological importance of waters properties and gets them thinking on the molecular level. Students immerse a Temperature Sensor into three different liquids; water, rubbing alcohol, and vegetable oil. They then graph the cooling curve once the probe is removed. The intermolecular forces play a big role in the energy it takes for the compounds to change state. Ask your students to do some research and explain how these forces connect with their data, or do some macro-scale modeling with a quick game of red-rover! The student version of the lab is available below.
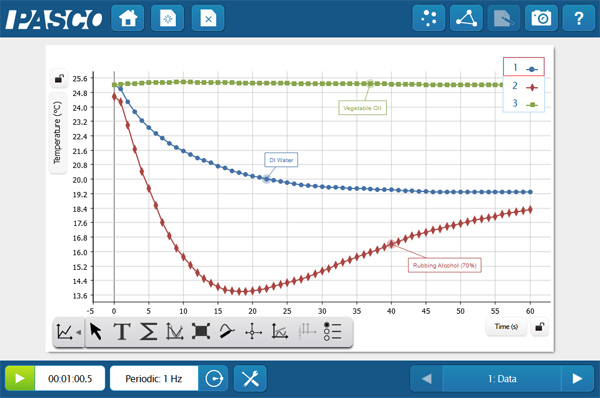
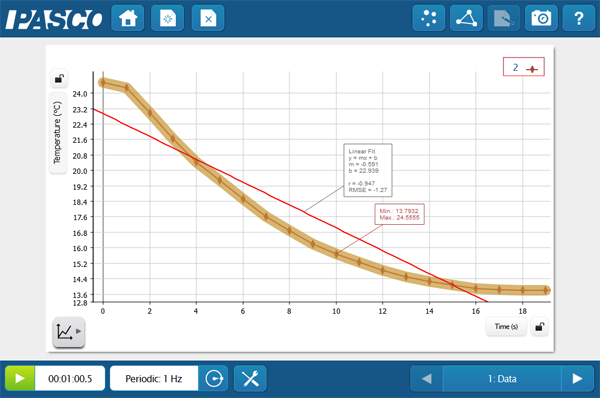
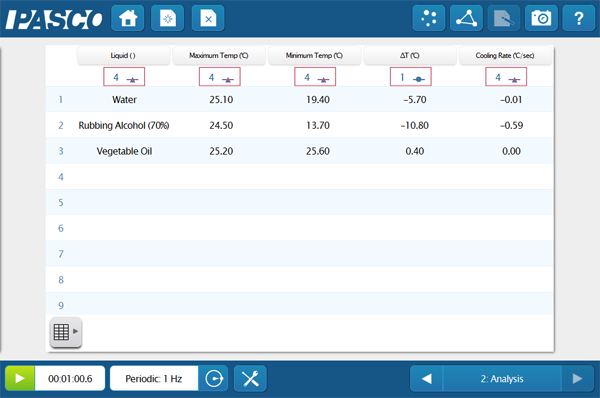
Students could easily expand the investigation to other materials to create an inquiry. Depending on the student level you can also enhance the analysis questions and challenge students to explain the evolutionary significance of this adaptation, or dig further into the chemical properties of each compound.
For Students:
You’re probably familiar with the effects of perspiration on the skin. It cools you off during a run, or on a hot summer day. But why does perspiration make you feel cool? Let’s investigate.
Materials:
- Data collection interface
- Stainless Steel or Fast-Response Temperature Sensor
- Cotton balls (2)
- Small cups or beakers with the following:
- Rubbing alcohol
- Water
- Vegetable or olive oil
Procedure:
- Build a graph display of Temperature vs. Time.
- Set the sample rate to 1/sec for 60 seconds.
- Place the tip of the Temperature Probe into the cup with water in it.
- After ~30seconds have one person press start while the other person simultaneously removes the probe from the liquid. Data collection will stop automatically.
- Repeat steps 2-4 with the other liquids after cleaning the probe.
Analysis:
- Complete the data table (linear regression optional)
- Record any qualitative observations about the liquids. What’s different about them?
- With the cotton ball place a little water on the inside of one elbow and a little rubbing alcohol on the inside of the other. What differences do you notice? Does your feeling match the data?
- What’s happening as the liquid evaporates? What accounts for the differences between them? Use your data to justify your explanation.
- What differences would you notice if you perspired alcohol instead of water?




