Chemistry in a Cuppa
Every January educators from across the UK gather for the Bett conference to learn about innovative ways to use technology in education. This year this lucky chemist had the opportunity to travel across the pond to London to participate and highlight some of the great new technology PASCO has to make technology integration easy and affordable. After riding the tube, saying hello to the queen and checking out some sites, I acclimated myself to the British way of life by get myself a cuppa.
Like any self-respecting chemist, I was traveling with my wireless pH sensor. It’s always the right time for some chemis-tea, so, I decided to do a little investigation to see how the pH changes as tea is prepared. And you can do this experiment with your students without even having to hop across the pond!
First up I needed a pH baseline so I measured the pH of of a glass of water. Then in succession I measured the pH of milk, tea and finally the tea with milk (no sugar, thank-you-very-much).

In addition to minding the gap, now it was time to mind the data.
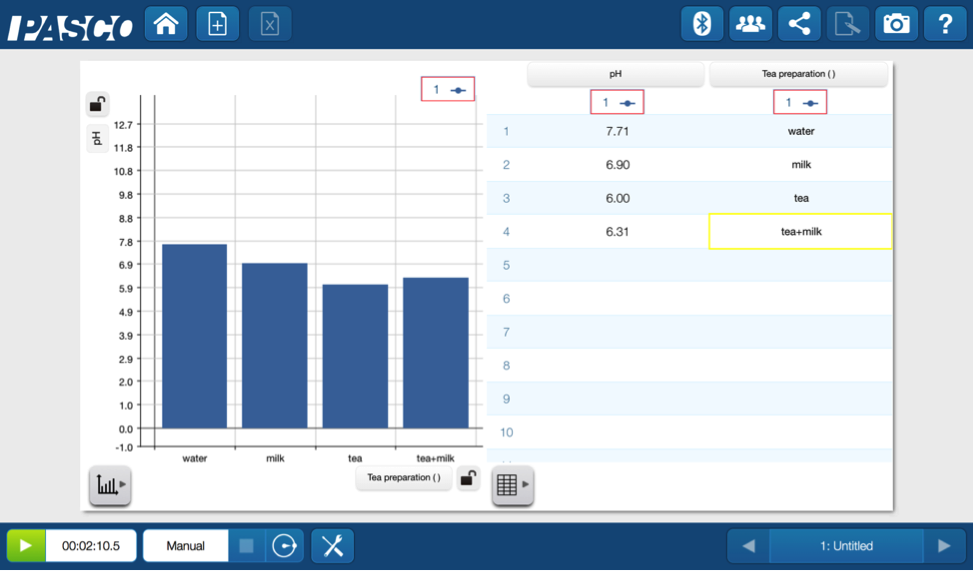
I assumed the water would be close to 7, but it was definitely not spot-on. The milk was slightly acidic because of the lactic acid. The tea, with a lower pH, was even more acidic than the milk. Of course the the addition of the milk to the tea pushed the pH back up.
Questions about the compounds that make up tea should begin to brew in your student’s minds— and as teachers we know that inspiring curiosity among students is even more valuable than the Crown Jewels . Because of the data they collect, they can meaningfully inquiry into the invisible phenomena that makes up the world around them.
Tea actually contains a wide variety of chemical compounds including polyphenols, which are thought to be responsible for a tea’s taste, color and reported antioxidant properties.
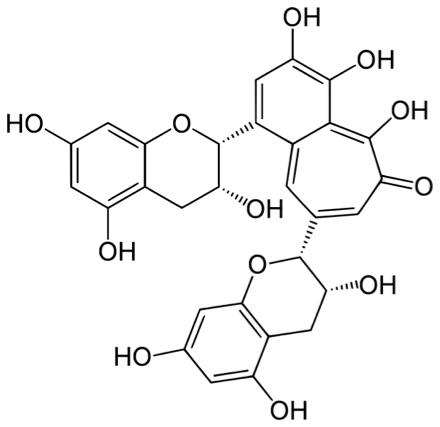
In addition to the polyphenols, tea can also be a significant source organic acids, like oxalic acid and malic acid, which would lower the pH.
Now that you students have a had a taste of the data, they have the opportunity explore more about what makes the perfect cup of tea.
Some investigative avenues could include:
- The pH of different types of teas
- The effect of brewing time on pH
- The molecular formulas and structures of polyphenols commonly found in tea (like theaflavin and catechin)
- The structure and properties of organic acids in tea (oxalic acid, maleic acid) and milk (lactic acid)
After an exhaustive study, it was time for this chemist to sit back and enjoy my chemis-tea and my delicious custard tart while I catch up with the latest happenings on Downtown Abbey. Cheers!


